CuSO₄ + 2NaOH = Cu(OH)₂ + Na₂SO₄
m(CuSO₄) = 40 г
m(Cu(OH)₂) = x
Mr(CuSO₄) = 160
Mr(Cu(OH)₂) = 98
m(Cu(OH)₂) = г
CuSO₄ + 2NaOH = Cu(OH)₂ + Na₂SO₄
m(CuSO₄) = 40 г
m(Cu(OH)₂) = x
Mr(CuSO₄) = 160
Mr(Cu(OH)₂) = 98
m(Cu(OH)₂) =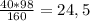 г
г