CaCO3=CaO+CO2
n CaO=50/56=0.893 моль
n CO2=0.893 моль
V CO2=0.893*22.4=20 л
m CO2=20*1.97=39.54г
50 x1\x2 56 22.4\44x1(V) = 22.4*50/56 = 20 л x2(m) = 44*50/56 = 39.2 гр
CaCO3=CaO+CO2
n CaO=50/56=0.893 моль
n CO2=0.893 моль
V CO2=0.893*22.4=20 л
m CO2=20*1.97=39.54г
50 x1\x2
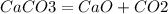
56 22.4\44
x1(V) = 22.4*50/56 = 20 л
x2(m) = 44*50/56 = 39.2 гр